

In the body, water moves through semi-permeable membranes of cells and from one compartment of the body to another by osmosis. In the human body, solutes vary in different parts of the body, but may include proteins-including those that transport lipids, carbohydrates, and very importantly, electrolytes. The dissolved substances in a solution are called solutes. The chemical reactions of life take place in aqueous solutions. Part 1: Body Fluids and Fluid Compartments Specify at least two causative disorders or circumstances that would cause each of the following and explain how they do so.Define alkalosis and acidosis, and specify two general causes of each.Describe acidosis and alkalosis in the body.
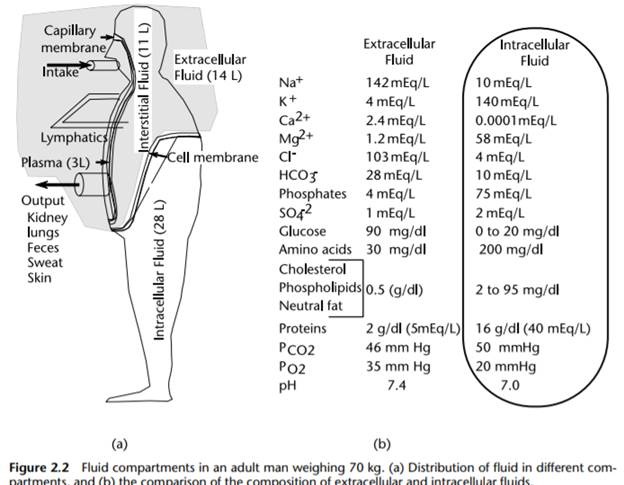
Describe the carbonic acid-bicarbonate buffer system and specify two other buffer systems.Describe three mechanisms by which the body regulates its pH.Introduction of regular foodstuffs into the digestive tract.Describe the mechanism(s) by which acids and/or bases arise in the human body as a result of each of the following:.Describe the function of such solutions in the human body and explain how they perform that function. Define the term buffer and explain the chemical nature of a buffer solution.III. Describe the mechanisms for the control of electrolytes in the body. Of the three major fluid compartments in the human body (intracellular fluid, blood plasma, and interstitial fluid), which two are most similar to each other? Explain why this grouping is not unexpected.Intracellular fluid and interstitial fluid.Compare and contrast the electrolyte composition of each of the following pairs of body fluid compartments:.
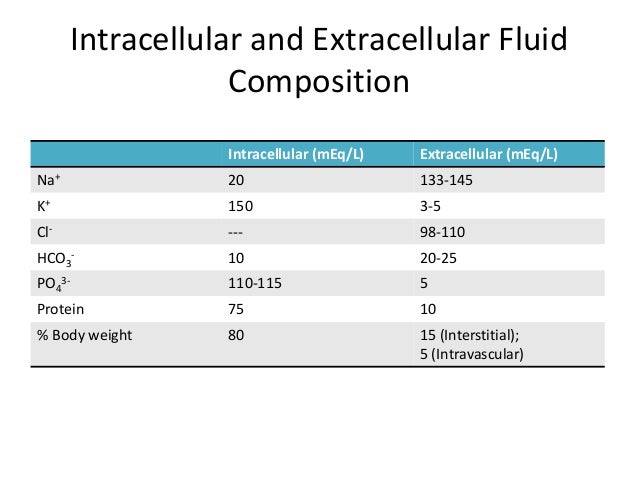
II. Describe the electrolyte composition of the body.
#Intracellular fluid composition how to#


 0 kommentar(er)
0 kommentar(er)
